Nuclear Energy
Nuclear energy is a tremendous source of energy. The energy is stored within the nucleus of atoms. According to Einstein’s famous formula: E=mc² the conversion of small amounts of matter releases huge amounts of energy.
It’s approximately 1 000 000 times(!) as energy dense as fossil fuels.
Just to give you an idea: the ball the person is holding is made of a metal called thorium. That ball contains all the energy that you need in all of your life, if you happen to be an average contemporary American. All of your flights, your HVAC, commute, lighting, cooking, everything. On a yearly basis, that thorium would cost you $1.
Given the vast potential for cheap energy inherent in nuclear power, I think it is an option worthy of consideration for combating climate change. This will be the first of a series of post about this topic.
There are two physical processes to release nuclear energy, these are called fusion and fission. In a fusion process, two nuclei are combined into a larger one. Amazingly, the resulting new nucleus is a little bit lighter than its two parents. The difference in mass is emitted via the kinetic energy in neutrons, as charged particles or as photons.
This process powers the stars. It’s working on an unimaginable large scale in the universe. It has been running for a few billion years and it will keep on doing so.
Fission is the reverse process. It starts with a heavy nucleus, splits it and ends up with lighter daughter nuclei. While there are natural fission processes, like the famous Oklo natural reactors or within liquid layers of our earth, it’s by far not as common as fusion.
One reason for that is that you need to have heavy elements to begin with. We currently believe that almost all of the heavy elements originate from super novae, the violent death of stars, or from colliding binary neutron stars. By contrast, hydrogen constitutes 70% of all the mass and 91% of all atoms in our solar system.
Basics
Chemical elements have isotopes. That means that the nucleus of the atom has the same number of positively charged protons, but a variable number of neutrons. For practical purposes, isotopes of the elements behave chemically identically; there is no difference in their reactions with other elements. But they are physically different, because they have different masses. Of the approximately 3300 different isotopes we know, only 240 are stable. All others are unstable: they decay and are therefore radioactive.
Hydrogen for example has three isotopes: the plain old 1H, also called protium, 2H, also called deuterium and 3H, called tritium. Deuterium constitutes 0.02% of natural occurring hydrogen atoms. Protium and deuterium are stable; tritium is unstable and radioactive. It has a half-life of approximately 12 years.
This means that every 12 years 50% of all tritium decays. Every 120 years, the amount of tritium is reduced to 1/1000th of the initial amount.
You can gain energy by the fusion of elements up to iron and reversely by the fission of heavier elements down to iron. The possible release of energy is depicted in this figure:
By looking at this figure it becomes apparent that fusion energy is the more attractive option; there are more atoms to be fused an there is more energy per atom to be gained. Why don’t we use this process all the time? Because it’s hard. Like really, really hard.
Inside the core of stars there are immense pressures: they compress hydrogen to almost ten times the density of gold. And it is hot: millions of degrees Celsius.
We can’t produce these pressures on earth.
But we are able to produce high enough temperatures to still make fusion work.
We actually know how to make that happen: modern thermonuclear bombs tap into this process. We are working on utilizing the same process in a less violent manner.
Fission
Fission on the other hand offers a short cut. If you have enough of the right material in just the right geometrical configuration, you can create a self-sustaining reaction. You need so called fissile materials for this to work. These materials include 233U, 235U and 239Pu. If you bombard these elements with neutrons of just the right amount of energy, they will split into lighter elements. The distribution of these fragments is somewhat random: you cannot predict into what fragments an individual nucleus will be shattered from a neutron, but the odds a known.
The important thing: among the fragments are again neutrons. In fact there are more than one on average. These can split new nuclei and so on.
The number of reactions and neutrons will grow quickly and a lot of energy can be released. In nuclear reactors, a lot of care is taken to ensure that the number of fission events peters out at a desired level. There a physical process that ensure a negative feedback. For example, if the rate of reaction grows, the temperature of the fuel rises. This leads to thermal expansion, which changes the geometry enough to guarantee that the rate of fission events is reduced.
In nuclear weapons on the other hand, a lot of work is put into the removal of all impediments to this chain reaction.
Moderators
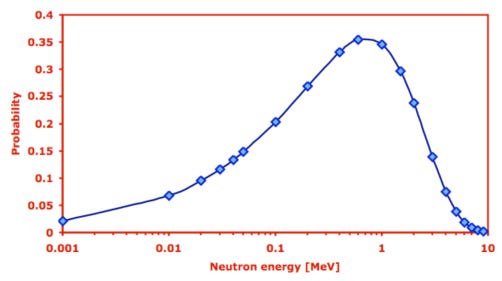
The neutrons that are generated by the fission of each fissile nucleus have a specific distribution of energy. Here is an example of how it looks like for 235U:
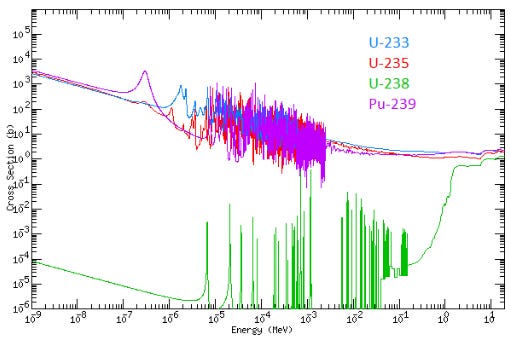
The probability that a neutron hits and splits a nucleus is a function of the neutron energy. As a rule of thumb for fissile materials: the slower the neutrons, the higher the probability that they will fission the nucleus. This can be seen in the following figure in one of the many special units of measurement in the nuclear space: the “barn”. This seems to have been a joke on “as big as a barn door” for something that is only a tiny 10-24 cm² area. The larger the area in “barn” the higher the probability that a neutron will fission the nucleus:
A higher fission probability per neutron means that you don’t need as much fissile material in the reactor to keep the reaction going.
There are two ways to handle this: either you work with the neutron spectrum you got, which is called a fast reactor type. This forces you to put a lot of fissile material in the reactor. The other way is to slow down the neutrons and get to something, that’s called a thermal reactor.
The materials that slow neutrons down, i.e. “cool” them, are called moderators. The most common materials for that are ordinary water, graphite and heavy water, where deuterium instead of protium forms the water molecules.
In essence, neutrons bump into these moderators and transfer some of their kinetic energy to them, without being absorbed. This figure should give you an idea about how a moderator influences the neutron energies:
The moderation qualities of materials differ quite a bit, which has real effects on the capabilities of reactors. Uranium found in nature, consists of 0.7% of 235U and 99.3% 238U. A heavy water moderator reactor, like the CANDU can use natural Uranium directly as fuel. Light water on the other hand is not as good a moderator. But it’s orders of magnitude cheaper. Large light water reactors need on the order of 3-5% of fissile material in their fuel to operate. Fast reactors and a lot of smaller thermal reactor designs need on the order of 15-20% fissile material.
There are more advantage to using moderators. For example, if you use water to slow down your neutrons to keep the nuclear reaction going, you can simultaneously use that water as coolant and even as working medium. Steam cycles are a mature technology with well developed, competitive supply chains. A fast reactor on the other hand has to use coolants that don’t slow down the neutrons too much. For that reason liquid metal, especially sodium, lead and a lead-bismuth mixture have been used. Helium as gaseous coolant and certain molten salts have also been proposed as coolants.
A lot of the components for these have to be specifically developed, which adds to the price tag of these reactors.
Fission Fuel
The vast majority of commercial reactors are large light water moderated reactors that rely on enriched uranium as their fuel. Given that 235U is the only fissile material found in any practical amounts in nature and that you can’t extract it chemically, because it behaves too similar to 238U, you need to enrich uranium by physical processes. Most commonly, you turn uranium into UF6, a gas, and use centrifuges to separate the lighter 235UF6 from the heavier 238UF6 via centrifugal forces. But there are also processes that use lasers that disproportionately excite 235UF6 and make it electrically separable.
All reactors create new isotopes during their operation. Specifically, reactors running on uranium always create some amount of plutonium.
It’s possible to chemically separate plutonium from uranium in the fuel that has already been used in a reactor, a process called reprocessing. A mixture of Pu/U can be used as fuel for light water reactors. A whole lot of Soviet nuclear weapons have been used in American commercial reactors in this way. The ultimate “swords to ploughshares” program.
Breeding
Some concepts that try to reduce costly enrichment and reprocessing as much as possible rely on the fact that there are are so called fertile materials. Fertile materials can become fissile, if they absorb a neutron.
To offer an analogy, fissile materials are like tinder, fertile materials are like wet wood. It takes some tinder to get the fire started. After that, you can place some of the wet wood around the fire to dry it. It’s possible to dry enough wet wood to keep the fire burning by moving the dried logs into the center of the fire and add more wet wood on the periphery to dry it. More astonishingly, it is possible to dry enough wood to start another fire with it. If you are running a reactor in a mode that’s creating more or at least as much fuel as it is using, that reactor is called a breeder. If it consumes exactly as much fuel as it creates, it is called an iso-breeder.
238U and 232Th are such fertile materials.
238U can capture a neutron an become 239Pu, 232Th becomes 233U by the same mechanism. They are the wet wood that can be dried.
To stretch the campfire analogy a little bit, you need to burn the fire at precise temperatures to dry these different sorts of wood effectively.
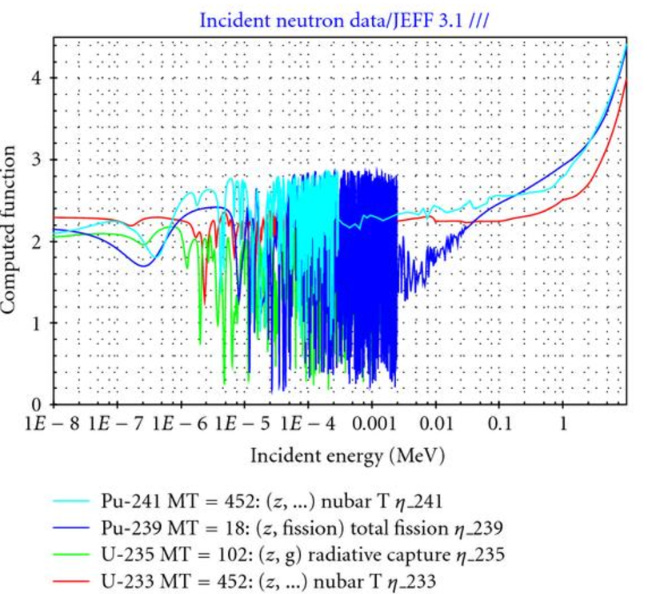
The U/Pu fuel cycle has to run “hot”. It relies on fast reactors. The reason can be seen in this plot:
239Pu doesn’t release more than 2 neutrons in huge segment of the “cold” spectrum on the left side.
But you need one neutron to breed new material and one to keep the chain reaction going. If you produce less than two neutrons per reaction, you are not drying enough wood and your fire fizzles out. Add in some losses and you will need even a little bit more than 2 for practical designs.
A U/Th breeding cycle on the other hand, can work in the thermal spectrum. 233U produces sufficient 2.3 neutron in the thermal spectrum.
There have only been a few demonstrations of breeding reactors, yet. Most experience has been gained with liquid metal cooled fast reactors. It has proven difficult to commercialize these reactors. Besides technical challenges regarding corrosion and neutron damage, pumps, seals, fuel shuffling for homogeneous burn-up, energy conversion cycles etc., there are also concerns about the high levels of fissile materials, especially plutonium.
Challenges
Reprocessing and enrichment are considered risks. These processes are geared at producing concentrated fissile material. And you need fissile materials to build nuclear weapons.
Centrifuges that you need to enrich your uranium to 3-5% 235U for light water reactor fuel could be used, daisy-chained together, to produce 93-95% enriched uranium, which you need for weapons. The plutonium that you extract from spent nuclear fuel could be diverted as well, although you will have to operate a reactor in a specific way to get a usable isotope distribution. Fast reactors (and also small thermal reactors) are especially prone to this fear, because they need on the order of 20% enriched fuel to operate.
Another risk of nuclear energy is the spent fuel itself. A lot of the fragments of the fission reaction are radioactive themselves and decay with rather short half-lives. The radiation is intense enough to severely harm or kill people that get exposed to it directly.
A few meters of water or thick enough lead or concrete are enough to bring the radiation down to well below normal ambient levels of radiation (from decaying elements in stone, cosmic radiation, etc.)
Most of the debate about nuclear waste is concerned with the long-lived elements that get created. These remain radioactive for a long period of time. Depending on the defined threat thresholds, these have to be safely stored for hundreds of thousands of years.
An obvious challenge concerns the cost of nuclear power plants. While there are certain burdens from the regulatory process, a huge problem comes from the fact that there typical power plants are so huge and slow to build that they are essentially always a “first-of-a-kind” product, which costs significantly more than a “nth-of-a-kind” product.
A lot of contemporary commercial efforts are geared towards modularization and downsizing of reactors. It’s hoped that the smaller components can be built in factories instead of being built on the construction site.
Lastly, the most important challenge for fission reactors is to make them reasonably safe to operate in all normal and accident condition. The irradiated nuclear fuel itself is one of the biggest problems. After shutting the reaction down, the fuel still produces on the order of 10% of the power from decaying radioisotopes. This decay heat is reduced to approximately 1% after a day.
Depending on the reactor design, this heat has to be removed actively, which is a problem in certain accident scenarios. The Fukushima power plant got destroyed by failing to cool the cores.
The reactors should also be reasonably robust with respect to intentional sabotage or even attacks.
Reactor types
There is an amazingly wide range of possible reactor designs and I will have a look at some of them in future posts. The following table gives an impression of the most important design parameters and the available options:
Fusion
Fusion power plants are generally considered safe from an operations perspective. Confining the fuel plasma in a configuration allowing fusion is so difficult and delicate that any disturbance will simply shut the plant down.
While there are various fuel options available, the vast majority of research has focused on “Deuterium-Tritium” fuel. The reason for that can be seen in the figure below; it’s the “easiest” option. This means that you “only” need to achieve 150 million °C.
As already mentioned, there is hardly any natural tritium. There is, however, a well known process to create tritium. If you bombard 6Li with neutrons, it fissions into tritium and 4He.
The deuterium-tritium fusion releases approximately 80% of its energy in the form of highly kinetic neutrons, some of which can be used for the breeding reaction. The remaining neutrons are slowed down in a coolant, which drives an ordinary steam cycle.
A 800MWe fusion power plant will use about 300g of tritium a day.
While the release of neutrons is great in terms of creating enough tritium to keep the reactor running, there is nothing really stopping you from using some of them to breed fissile materials. Just put some 238U in the reactor building of the fusion power plant and you will breed significant quantities of plutonium, which is a proliferation concern. These concerns are heightened by the fact that 6Li is used in thermonuclear bombs, where the deuterium-tritium fusion process is used in a destructive fashion.
There are some fusion reactor designs that try to use the H11B fuel. It’s widely available and releases hardly any neutrons. The process has two steps. First the hydrogen and the boron are fused to from 12C, which then fissions almost instantly into 3 helium ions. In a sense, this process is yet again a fusion-fission hybrid, like the deuterium-tritium cycle that relies on fission for its fuel. Because most of its energy is released in the form of charged particle, these can be slowed down in a “reverse-particle-accelerator” to produce electricity directly without the need for a steam power cycle. The downside: you need on the order of 1.5 billion °C to run the reaction.
We will look at the thriving field of entrepreneurialism that is fusion in future posts.
Summary
The big challenges for nuclear energy are:
fuel, safety, proliferation, waste … and of course economics. Nuclear power will have to compete with fossil fuels and cooperate with renewable energies to make a dent into global energy consumption patterns.
We will talk about all of these challenges in the future.
But I like to state here that I think the economics are the most problematic hurdle to overcome. A price on carbon would help tremendously.
But luckily, people are feverishly working on the technology itself. We owe them a big thank you!